The Electropolishing Process
Benefits of Electropolishing
Electropolishing enhances the corrosion resistance, luster and smoothness of many metals, particularly the 300 series stainless steels. Equally good results may be obtained on the 400 and precipitation hardening series, Inconel, Hastelloy and other nickel-based alloys.
Corrosion Resistance: By effectively removing impurities, electropolishing leaves a homogeneous metal surface. Furthermore, a high chrome/iron oxide ratio can be achieved which is of particular importance in UHP applications as discussed in a separate section. As a result, the inherent corrosion-resistance of such alloys as stainless steel is greatly improved. Salt spray tests have proved that electropolished parts are four to five times more resistant to corrosion than untreated parts.
Microfinish: The normal electropolishing process improves the microfinish by a factor of 2, i.e. 50 Ra is reduced to 25 Ra. With special processing, this can be reduced even further. The smoothness obtainable has several applications: where antifriction and non-galling are requirements such as in the production of gears and valves; where release properties are needed inside vessels or tubes; or where heat and light reflectivity is a factor.
Microsizing: The range of metal removal is normally from 0.000050 inch to 0.002 inch and electropolishing can be used as a method for sizing parts to close tolerances within 0.0001 inch. Although the removal rate is usually identical on all surfaces, selective removal is possible with proper techniques. Electropolishing is ideally suited for processing parts in quantity to produce uniform size or weight.
Preparation of Surfaces: As a pre-treatment for further processing, such as welding, plating or anodizing, electropolishing is used to remove the contaminated surface generated during manufacturing, leaving a smooth, chemically clean surface with superior adhesion qualities.
Deburring: Some parts are too heavy or fragile for tumbling, or have intricate shapes and deep recesses that are difficult to deburr mechanically. Electropolishing is the best answer to such deburring problems, and is usually less expensive than other methods.
Cleanliness: Electropolished surfaces are stain and bacteria resistant to meet the exacting cleanliness standards of the food processing, medical equipment and chemical industries.
Endurance: Cracks and other surface defects act as stress-concentration sites, and when removed by electropolishing the fatigue strength of a metal is improved. This applies particularly to springs, where tests have shown fatigue life to be greatly increased after treatment.
Appearance: A widespread use of electropolishing is for enhancement in the appearance of a product. No other finish can provide such a brilliant effect, and imperfections such as stains, heat discoloration, weld marks and minor scratches are eliminated or minimized.
Inspection: Mechanical-finishing operations often smear over the metal surface giving a deceptively smooth appearance, which will eventually deteriorate. Electropolishing reveals the true microstructure of the metal and thus becomes an effective inspection tool.
Technology
Electropolishing is accomplished by connecting the metal part to be processed to the positive terminal (the anode) of a DC power supply. The part is then immersed in a heated electrolytic bath that contains metal plates connected to the negative terminal (the cathode). The electrical reaction causes an ionic conduction resulting in the removal of particles of metal from the anode.
During the process, the products of this anodic metal dissolution react with the electrolyte to form a film at the surface of the metal. This film essentially conforms to the general contour of the surface of the metal and therefore is thinner over the micro-projections and thicker at the micro-depressions. The result is more rapid dissolution of the micro-projections causing micro-leveling at the surface.


The amount of metal removed is influenced by the composition of the metal part to be electropolished, the temperature and agitation of the electrolytic bath, the spatial and area relationships of the anode and cathode, the current, and the length of time the current is flowing.
The result of electropolishing is demonstrated in the following diagrams showing the effect on the surface of the metal.
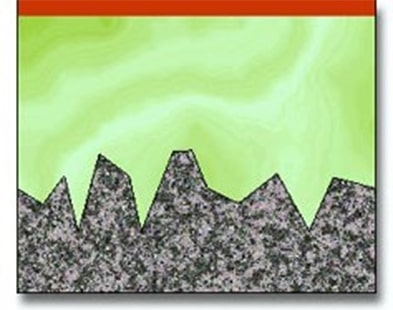
Surface profile of workpiece before electropolishing
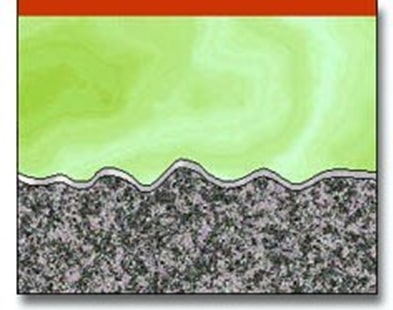
Action of electropolishing demonstrating leveling effect and chrome-enriched surface
Passivation
Passivation is the creation of an oxide layer of the base constituents of the CRES alloy. In the case of “standard” Stainless Steels such as 302, 304 and 316, this oxide layer consists of iron, chromium and nickel. The passive layer is thin, but it greatly enhances the corrosion resistance of the steel. The parts are cleaned of any surface contamination (greases, oil and even fingerprints), then the parts are submerged in an acid bath according to the type of steel, as instructed in the Passivation Specification, to produce the passive layer in short order. Areas of weld oxides and other imbedded contaminants are removed prior to the passivation bath to insure a uniform passive layer on the entire part.
Electromatic is well versed in this process and thoroughly equipped to perform passivation on a variety of products. These range from small cable assemblies to enormous tanks and effluent output troughs, such as for the City of Los Angeles Wastewater Treatment facility. Electromatic is also equipped and able to perform local on-site passivation of any project size. We have done on-site passivation for large projects at Disneyland Resort and Universal Studios Hollywood.
Electromatic strictly conforms to the following specifications in passivating parts, QQ-P-35A (the original Federal Specification for Passivation Treatments of Corrosion Resistant Steels – 1973), ASTM A967© – Standard Specification for Chemical Passivation Treatments for Stainless Steel Parts, ASTM B912© – Standard Specification for Passivation of Stainless Steel Parts using Electropolishing, SAE-AMS 2700© – Passivation of Corrosion Resistant Steels and ASTM A380© – Standard Practice for Cleaning, Descaling and Passivation of Stainless Steel Parts, Equipment and Systems.
Other Operations
Various other metal finishing services are provided by Electromatic, including alkaline cleaning, pickling, bead blasting and brush electropolishing.